Image 1 of
Image 1 of


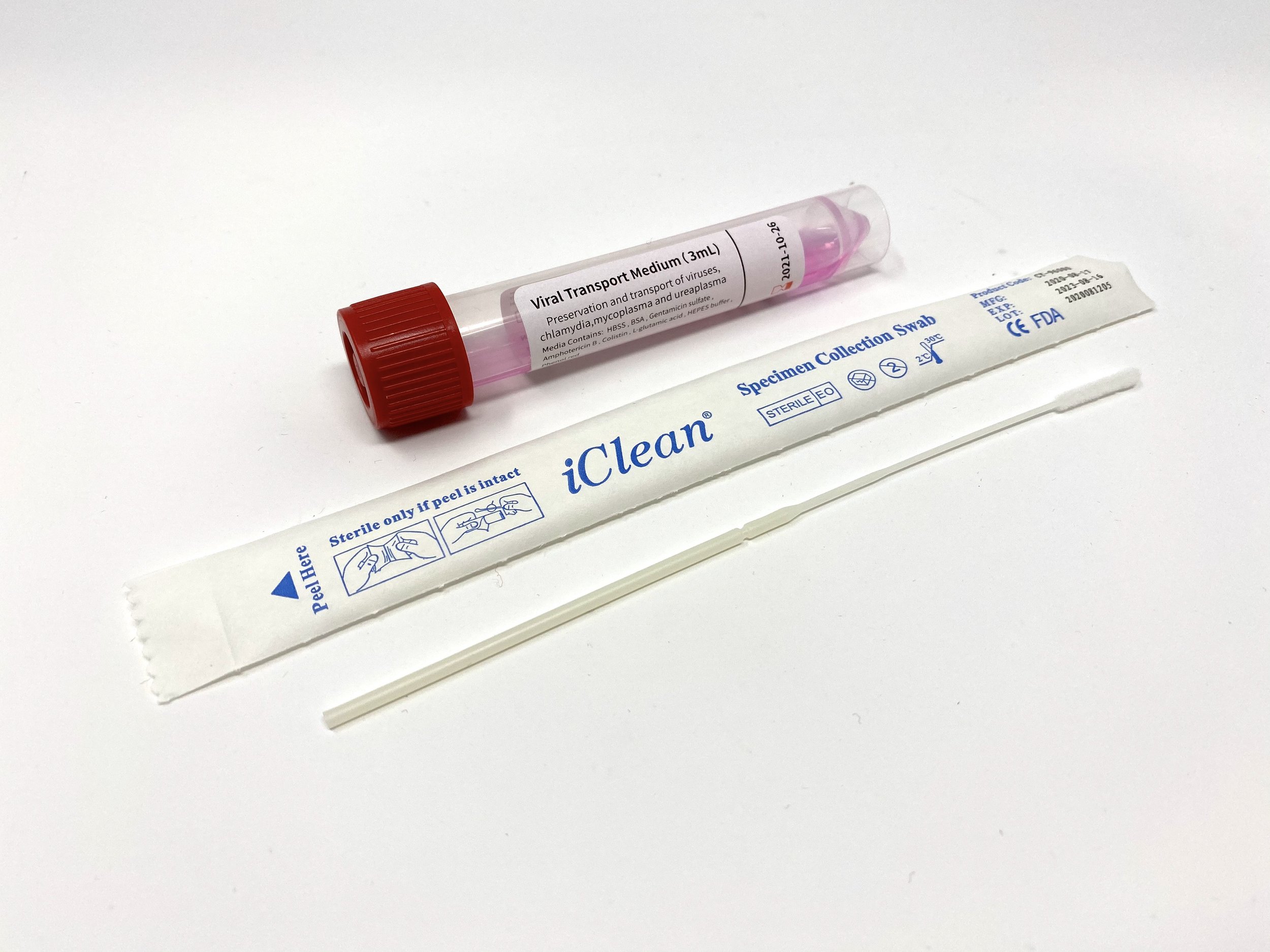
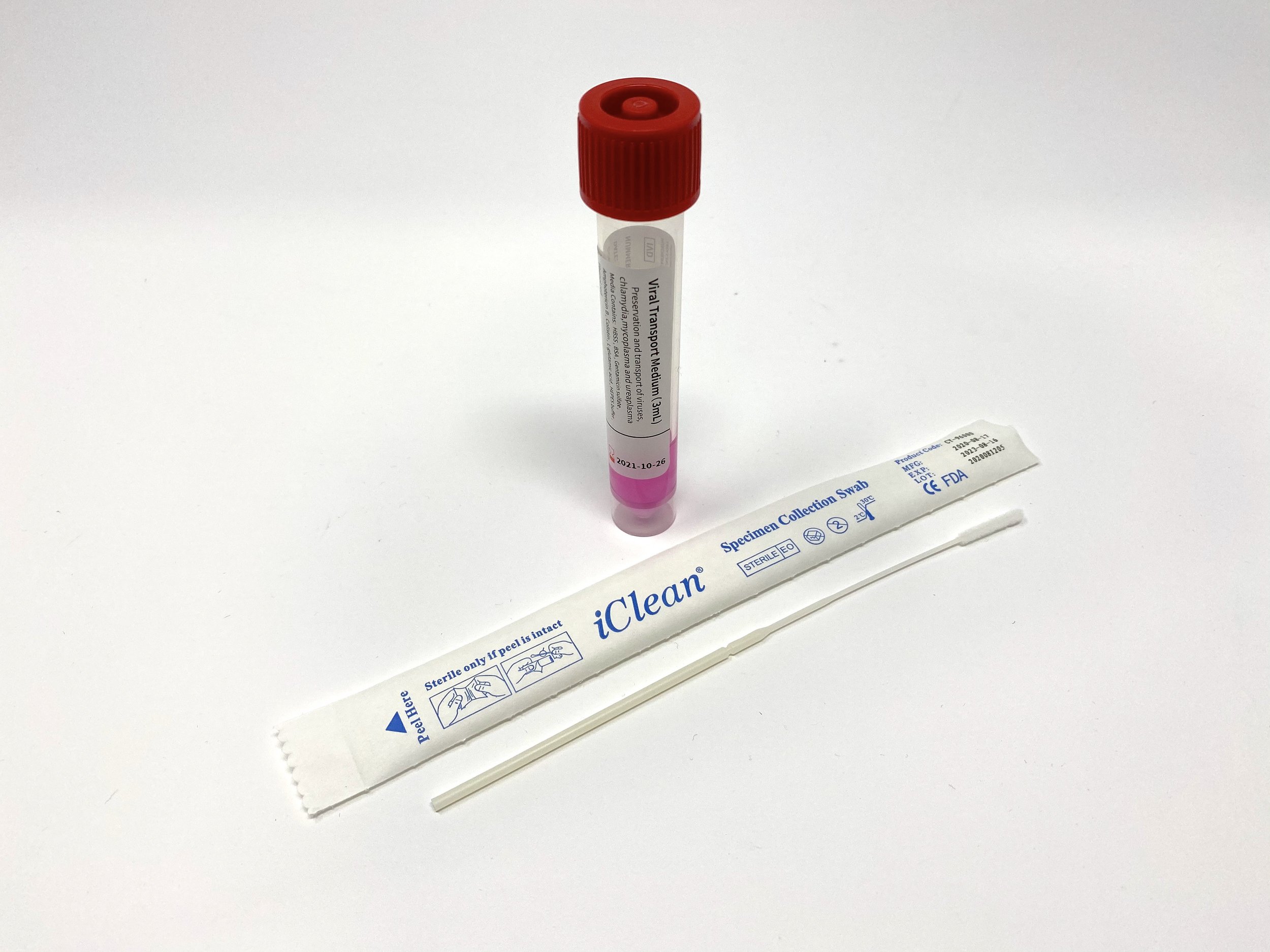
iClean® VTM
Now fully FDA 510k cleared. iClean® Swabs paired with Viral Transport Media. Our iClean® VTM kits are stocked here in California. Ships immediately.
Now fully FDA 510k cleared. iClean® Swabs paired with Viral Transport Media. Our iClean® VTM kits are stocked here in California. Ships immediately.
Now fully FDA 510k cleared. iClean® Swabs paired with Viral Transport Media. Our iClean® VTM kits are stocked here in California. Ships immediately.
Specifications
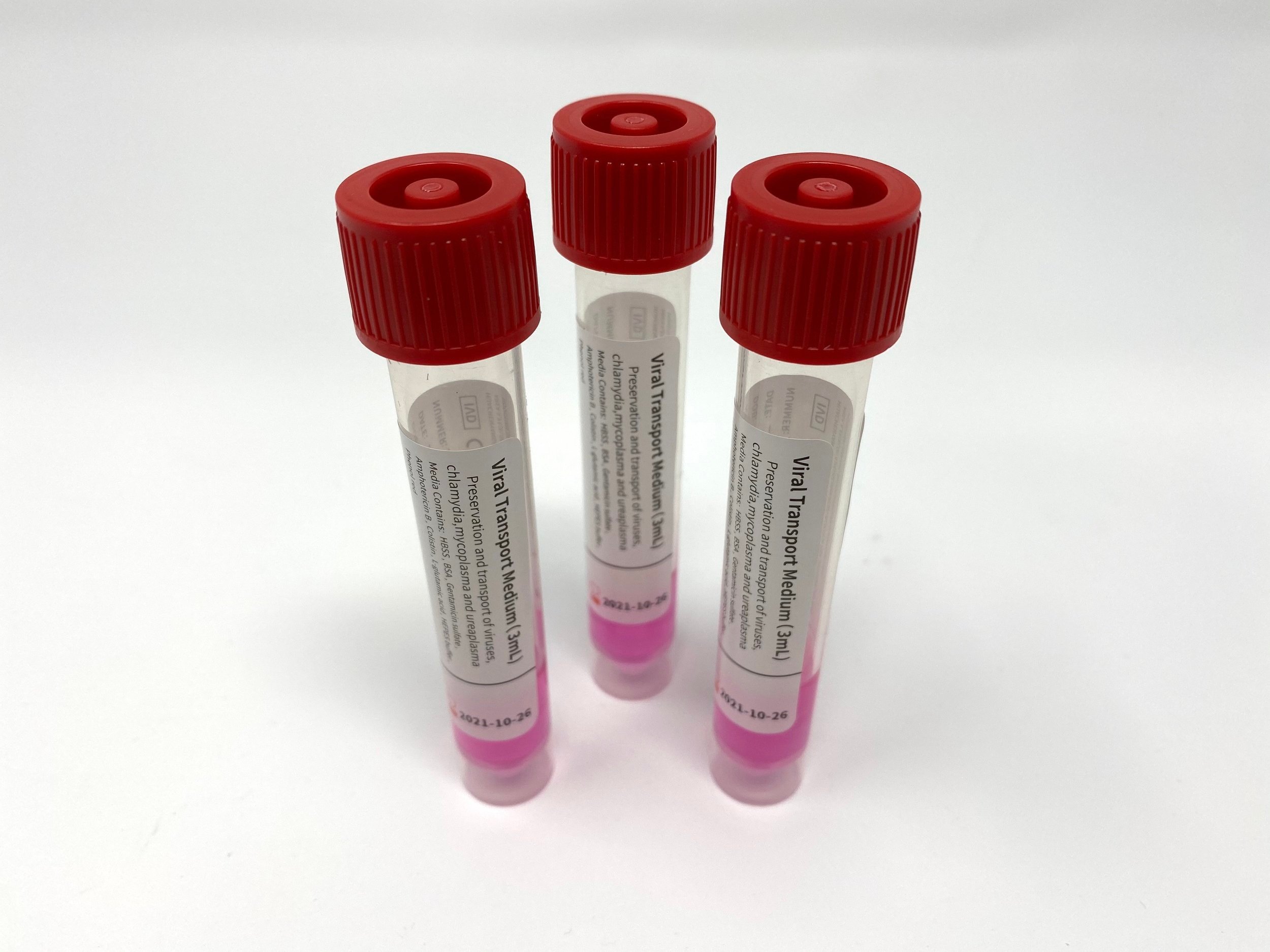
Tube Type: 10mL polypropylene plastic tubes with screw cap
Swab Types: Medical grade, nasopharyngeal or oropharyngeal nylon flocked swabs.
Volume of Media: 3.0 mL
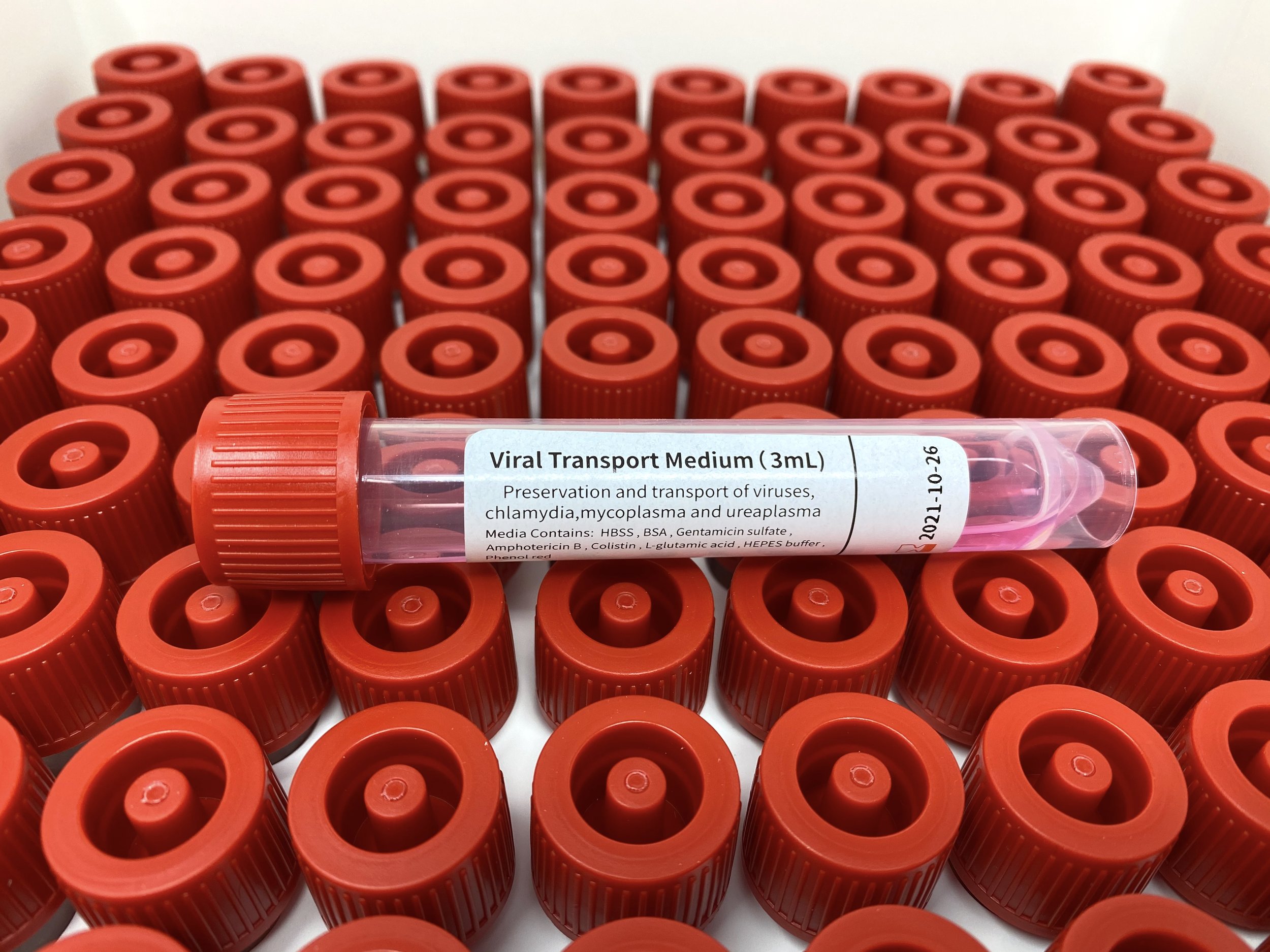
Transport Media Type: Universal transport media, sterile, made with salts, antibiotics, preservation agents for viruses, chlamydiae, ureaplasma, and mycoplasma, and collection tube.
pH of Media: 7.1-7.5
Specimen Storage Temperature: Room or cold temperature, 2-25°C
Media Type & Composition:
Sterile viral transport media with pH indicator, antibiotics, and collection tube, includes:
Hank’s Balanced Salts Solution (HBSS)
Phenol Red
Bovine Serum Albumin (BSA)
HEPES Buffer
Gentamicin Sulfate
Vancomycin
L-Glutamic Acid
Colistin
Media and Virus Stability:
Unused media may be transported and stored at room temperature, 15°C - 25°C.
Shelf life of unused media is 12 months.
After collection, samples should be stored and transported at room or cold temperature 2 to 25°C for up to 48 hours.
If samples must be stored longer than 48 hours, it is recommended to cryopreserve them at -70°C.
Intended Use
For the collection, preservation, and transport of clinical specimens to a testing laboratory. Transport medium serves as a culture media for non-propagating transport of specimens.